Introduction: Unrelated cord blood transplantation (UCBT) and haploidentical stem cell transplantation with post-transplant cyclophosphamide as graft-versus-host disease (GVHD) prophylaxis (PTCy-haplo) are alternative options for patients with hematologic disease lacking a human leukocyte antigen (HLA)-matched donor. Non-relapse mortality (NRM) after UCBT may vary depending on the cord blood CD34+ cell count, but no study has accounted for CD34+ cell count when comparing the outcomes after UCBT and PTCy-haplo. Therefore, we analyzed transplant outcomes after UCBT and PTCy-haplo in patients with hematologic disease from data of a nationwide registry that included cord blood CD34+ cell count.
Methods: Patients with hematologic disease receiving first UCBT or PTCy-haplo between 2014 and 2020 were identified from the nationwide registration data of the Japanese Data for Hematopoietic Cell Transplantation. Acute GVHD-related mortality was defined as death caused primarily by GVHD or occurred secondary to GVHD, and infection-related mortality as death due to graft failure or infection but not acute GVHD. Patients treated by UCBT were stratified by the median CD34+ cell count for comparison (0.84 × 10 5/kg).
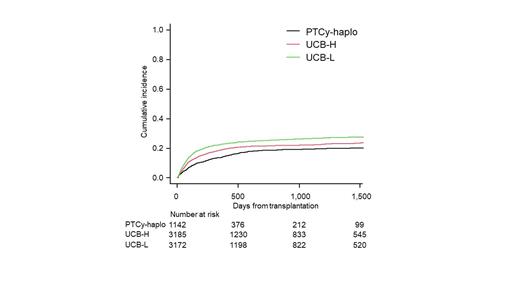
Results: A total of 7,499 patients were enrolled and classified into 3 groups: PTCy-haplo (n = 1,142), UCBT with high CD34+ cell count (UCB-H; ≥ 0.84 × 10 5/kg, n = 3,185), and UCBT with low CD34+ cell count (UCB-L; < 0.84 × 10 5/kg, n = 3,172). Patients in the PTCy-haplo group were slightly younger than UCB-H and UCB-L patients (median age: 54 vs. 56 and 55 years). The PTCy-haplo group also included a greater proportion of males than the UCB-H group (62.4% vs. 50.0%) but not the UCB-L group (64.2%). A greater proportion of PTCy-haplo patients had a PS score of 0 (53.2% vs. 46.2% and 45.6%), a hematopoietic cell transplantation-specific comorbidity index of 0 (57.6% vs. 52.7% and 54.4%) and donor-specific anti-HLA antibody (2.7% vs. 1.0% and 1.6%), received reduced-intensity conditioning (58.0% vs. 40.5% and 41.6%), calcineurin inhibitor with mycophenolate mofetil (88.5% vs. 38.6% and 38.4%), and underwent transplantation in the later period (82.0% vs. 54.6% and 50.7%). There were no significant differences in disease-risk index between groups. At 2 years post-transplantation, NRM and overall survival (OS) were significantly better in the PTCy-haplo group than the UCB-H and UCB-L groups (NRM: 18.8% vs. 21.6% and 25.3%, P < 0.001; OS: 56.2% vs. 53.3% vs. 49.6%, P < 0.001). Neutrophil engraftment rate at 42 days was also significantly higher in the PTCy-haplo group than the UCB-H and UCB-L groups (93.7% vs. 87.5% and 82.9%, P < 0.001). The PTCy-haplo group less frequently developed grade II-IV (26.4% vs. 37.3% and 34.4%, P < 0.001) and III-IV acute GVHD (7.0% vs. 12.2% and 10.5%, P < 0.001). While neither the differences in NRM nor that in OS remained significant between PTCy-haplo and UCB-H groups in multivariable analysis (NRM: hazard ratio [HR], 1.18, 95% confidence interval [CI], 0.99-1.41, P = 0.073; OS: HR, 1.05, 95% CI, 0.94-1.17, P = 0.42), significant differences were maintained between PTCy-haplo and UCB-L groups (NRM: HR, 1.35, 95% CI, 1.13-1.61, P = 0.001; OS: HR, 1.12, 95% CI, 1.00-1.26, P = 0.042). Regarding the cause-specific mortality, infection-related mortality was also significantly lower in the PTCy-haplo group compared to UCB-H and UCB-L groups (8.1% vs. 11.1% and 13.6%, P < 0.001) while GVHD-related mortality did not differ among groups (5.0% vs. 5.2% and 4.9%, P = 0.69).
Conclusion: UCBT-H resulted in similar NRM and OS as patients receiving PTCy-haplo, whereas outcomes were worse in UCBT-L. These findings indicate that high CD34+ cell count in cord blood is a critical criterion for alternative donor selection.
Disclosures
Harada:Nippon Shinyaku: Research Funding. Kanda:Amgen: Ended employment in the past 24 months, Honoraria; Janssen Pharmaceutical K.K.: Honoraria; Novartis Pharma K.K.: Honoraria; Sanofi K.K.: Honoraria; AbbVie Pharma: Honoraria; Megakaryon Co.: Honoraria; Eisai Co.: Research Funding. Nakamae:CMIC Company: Research Funding; Parexel International Inc: Research Funding; Takeda Pharmaceutical Company: Honoraria; Novartis: Research Funding; Alexion Pharma: Research Funding; Meiji Seika Pharma: Research Funding; DAIICHI SANKYO COMPANY: Honoraria; Janssen Pharmaceutical K.K.: Honoraria; Nihon Shinyaku: Honoraria; Sumitomo Dainippon Pharma: Honoraria; Bristol-Myers Squibb: Research Funding; Amgen: Honoraria; Otsuka: Honoraria; Abbvie: Honoraria; Astellas: Honoraria. Tanaka:Abbvie: Speakers Bureau; Asahi Kasei Pharma: Speakers Bureau; Astellas Phrama: Speakers Bureau; Chugai Pharmaceutical: Speakers Bureau; Daiichi Sankyo: Speakers Bureau; Kyowa-Kirin: Speakers Bureau; MSD: Speakers Bureau; Otsuka Pharmaceutical: Speakers Bureau; Pfizer: Speakers Bureau; Sumitomo Pharma: Speakers Bureau. Atsuta:Otsuka Pharmaceutical Co., Ltd: Speakers Bureau; Novartis Pharma KK: Speakers Bureau; CHUGAI PHARMACEUTICAL CO., LTD.: Speakers Bureau; Meiji Seika Pharma Co, Ltd.: Honoraria; JCR Pharmaceuticals Co., Ltd.: Consultancy. Nakasone:Otsuka Pharmaceutical: Honoraria; Bristol-Myers Squibb: Honoraria; Pfizer: Honoraria; Novartis: Honoraria; Takeda Pharmaceutical: Honoraria; Eisai: Honoraria; Chugai Pharmaceutical: Honoraria; Sanofi: Honoraria; Meiji Seika Pharma: Honoraria; Nippon Shinyaku: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal